Stability of the Key Substances in Isaria tenuipes Extracts in Cosmetic Products
DOI:
https://doi.org/10.53848/ssstj.v12i1.1012Keywords:
Adenosine, Cordycepin, Isaria tenuipes, Polysaccharide, StabilityAbstract
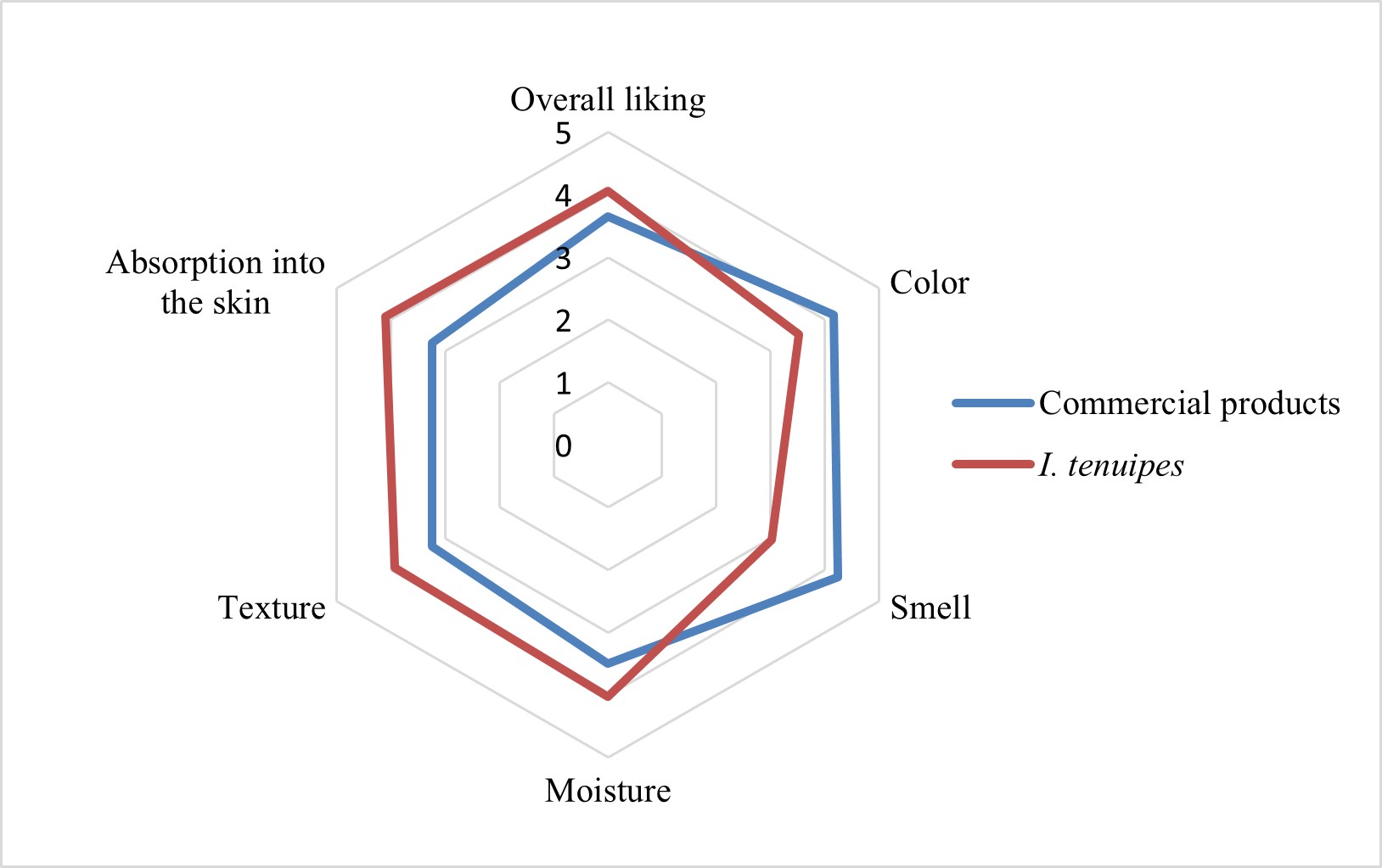
This paper quantified key bioactive substances in Isaria tenuipes extracts on a dry weight basis, including cordycepin (0.32 mg/g), adenosine (5.62 mg/g), and polysaccharides (65.8 mg/g). Antioxidant activity was evaluated using DPPH and ABTS assays, yielding IC₅₀ values of 0.27 mg/g and 0.04 mg/g, respectively. Stability testing demonstrated that polysaccharides and adenosine remained relatively stable (less than 10% degradation) after 12 weeks of storage at both 4°C and 30°C, whereas cordycepin exhibited a greater reduction. The optimal formulation for incorporating I. tenuipes extract into a serum product was identified as 2.0% (w/w). After 12 weeks of storage at 30°C, the primary active compounds in the serum showed minimal degradation (<10%), while the pH, viscosity, and color remained stable at 4°C. Microbiological analysis confirmed compliance with industrial safety standards. Consumer evaluation using a 5-point hedonic scale (n = 100) indicated high satisfaction with moisturization (4.02), texture (3.93), absorption (4.10), and overall preference (4.06), outperforming a commercial reference product. These findings highlight the stability and cosmetic potential of I. tenuipes extract-based formulations.
References
Antignac, E., Nohynek, G. J., Re, T., Clouzeau, J., & Toutain, H. (2016). Safety of botanical ingredients in personal care products/cosmetics. Food and Chemical Toxicology, 49, 324–341.https://doi.org/10.1016/j.fct.2010.11.022
Blasi, F., & Cossignani, L. (2020). An overview of natural extracts with antioxidant activity for the improvement of the oxidative stability and shelf life of edible oils. Processes, 8, 956. https://doi.org/10.3390/pr8080956
Bravo, C., Pena, F., Nahuelcura, J., Vidal, C., González, F., Jiménez-Aspee, F., Bustamante, L., Contreras, B., & Ruiz, A. (2023). Stability of phenolic compounds, antioxidant activity and color parameters in colored-flesh potato chips. Molecules, 28, 6047. https://doi.org/10.3390/molecules28166047
Chittasupho, C. (2016). Stability of drugs and pharmaceutical products. Triple Group Co., Ltd.
Choi, J. S., Heo, J. H., Kim, D. J., Namkung, S. M., Lee, T. B., Lee, M. W., & Kim, S. W. (2017). Anti-cancer effect of hot water extract from mycelium in germanium-enriched Cordyceps militaris. The Korean Journal of Clinical Laboratory Science, 49(2), 69–78. https://doi.org/10.15324/kjcls.2017.49.2.69
Dreywood, R. (1946). Qualitative test for carbohydrate material. Industrial and Engineering Chemistry Analytical Edition, 18(8), 499. https://doi.org/10.1021/i560156a015
Du, L., Song, J., Wang, H., Li, P., Yang, Z., Meng, L., Meng, F., Lu, J., & Teng, L. (2012). Optimization of the fermentation medium for Paecilomyces tenuipes N45 using statistical approach. African Journal of Microbiology Research, 6(32), 6130–6141. https://doi.org/10.5897/AJMR11.1624
Fernandes, P. A. R., & Coimbra, M. A. (2023). The antioxidant activity of polysaccharides: A structure-function relationship overview. Carbohydrate Polymers, 314, 120965. https://doi.org/10.1016/j.carbpol.2023.120965
Haghparast, S., Shabanpour, B., Kashiri, H., Alipour, Gh., & Sodagar, M. (2013). A comparative study on antioxidative properties of carameled reducing sugars; Inhibitory effect on lipid oxidative and sensory improvement of glucose carameled products in shrimp flesh. Journal of Agricultural Science and Technology, 15(1), 87–99.
Kang, P.-D., Sung, G.-B., Kim, K.-Y., Kim, M.-J., Hong, I.-P., & Ha, N.-G. (2010). Breeding of a silkworm variety for synnemata production of Isaria tenuipes. Mycobiology, 38(3), 180–183.
Kanlayavattanakul, M., & Lourith, N. (2012). Preparation of standardized Litchi peels of a prototype for a health product. CDIT Co., Ltd.
Li, J., Guan, M., & Li, Y. (2015). Effects of cooking on the contents of adenosine and cordycepin in Cordyceps militaris. Procedia Engineering, 102, 485–491. https://doi.org/10.1016/j.proeng.2015.01.195
Lourith, N., & Kanlayavattanakul, M. (2013). Antioxidant activities and phenolics of Passiflora edulis seed recovered from juice production residue. Journal of Oleo Science, 62, 235–240.https://doi.org/10.5650/jos.62.235
Manolopoulou, E., & Varzakas, T. (2016). Effect of temperature in color changes of green vegetables. Current Research in Nutrition and Food Science, 4, 10–17. https://doi.org/10.12944/CRNFSJ.4.Special-Issue-October.02
Munisekhar, M. (2014). Keratolytic composition with anti-allergic anti-inflammatory properties (U.S. Patent No. US20060241190A1). United States Patent and Trademark Office.
Pakpiangchan, P., & Chusuwan, T. (2018). Processing of the Cordyceps and the development of a prototype for a health product. CDIT Co., Ltd.
Piljac-Zegarac, J., & Samec, D. (2011). Antioxidant stability of small fruits in postharvest storage at room and refrigerator temperatures. Food Research International, 44, 345–350.https://doi.org/10.1016/j.foodres.2010.09.039
Prommaban, A., Sriyab, S., Marsup, P., Neimkhum, W., Sirithunyalug, J., Anuchapreeda, S., To-anun, C., & Chaiyana, W. (2022). Comparison of chemical profiles, antioxidation, inhibition of skin extracellular matrix degradation, and anti-tyrosinase activity between mycelium and fruiting body of Cordyceps militaris and Isaria tenuipes. Pharmaceutical Biology, 60(1), 225–234. https://doi.org/10.1080/13880209.2021.2025255
Ruen-ngam, D., Thawai, C., Sukonthamut, S., Nokkoul, R., & Tadtong, S. (2018). Evaluation of nutrient content and antioxidant, neuritogenic, and neuroprotective activities of upland rice bran oil. ScienceAsia, 44, 257–267. https://doi.org/10.2306/scienceasia1513-1874.2018.44.257
Sapkota, K., Moon, S., Choi, B., Kim, S., Kim, Y., & Kim, S. (2011). Enhancement of IL-18 expression by Paecilomyces tenuipes. Mycoscience, 52(4), 260–267. https://doi.org/10.1007/S10267-010-0101-4
Sharma, S. K. (2015). Optimized extraction and antioxidant activities of polysaccharides from two entomogenous fungi. Journal of Bioanalysis and Biomedicine, 7, 180–187. https://doi.org/10.4172/1948-593X.1000141
Southgate, D. A. T. (1969). Determination of carbohydrates in foods. I. Available carbohydrate. Journal of the Science of Food and Agriculture, 20(6), 326–330. https://doi.org/10.1002/jsfa.2740200602
The Central Lab by Pathawin. (2020a). Report on microbial test (USP 41, chapter 61).http://www.thecentrallab.com/
The Central Lab by Pathawin. (2020b). Report on microbial test (USP 41, chapter 62).http://www.thecentrallab.com/
Wang, Y., Xu, F., Cheng, J., Wu, X., Xu, J., Li, C., Li, W., Xie, N., Wang, Y., & He, L. (2022). Natural deep eutectic solvent-assisted extraction, structural characterization, and immunomodulatory activity of polysaccharides from Paecilomyces hepiali. Molecules, 27, 8020. https://doi.org/10.3390/molecules27228020
Wu, Y., Choi, M., Li, J., Yang, H., & Shin, H. (2016). Mushroom cosmetics: The present and future. Cosmetics, 3, 22. https://doi.org/10.3390/cosmetics3030022
Wu, Z., Zhang, M., Xie, M., Dai, Z., Wang, X., Hu, B., Ye, H., & Zeng, X. (2016). Extraction, characterization, and antioxidant activity of mycelial polysaccharides from Paecilomyces hepiali HN1. Carbohydrate Polymers, 137, 541–548. https://doi.org/10.1016/j.carbpol.2015.11.010
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Suan Sunandha Rajabhat University

This work is licensed under a Creative Commons Attribution 4.0 International License.











