The Carotenoids Production from Rhodopseudomonas sp. OS33-UV13-5 Cultivated in Chitin Medium
DOI:
https://doi.org/10.53848/ssstj.v12i2.1013Keywords:
Chitin, Carotenoids, Rhodopseudomonas sp, N-acetyl glucosamineAbstract
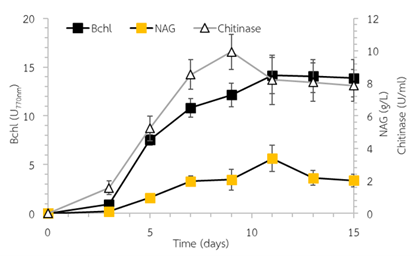
In this work, the carotenoids (CD) production of Rhodopseudomonas sp. OS33-UV13-5 in the presence of different chitin forms such as chitin powder, chitin flake, squid pen and squid pen powder was studied for carbon source utilization, respectively. Rhodopseudomonas sp. OS33-UV13-5 showed the ability to degrade chitin. When Rhodopseudomonas sp. OS33-UV13-5 was cultivated in chitin medium containing 10 g/L of squid pen powder as a carbon source, the growth (bacteriochlorophylls content, Bchl) and CD were found at 6.32±0.75 Bchl U770nm and 23.14±1.86 mg/L, respectively. Then, the optimized culture conditions were examined with various squid pen powder concentrations and light intensities. The results showed that maximum cell growth and CD production were 14.64±1.91 Bchl U770nm and 45.87±3.85 mg/L, respectively, when cultivated in 30 g/L of squid pen powder under 1500 lux of light intensity at 40oC for 15 days. The CD production increased by about 2.02-fold. The Rhodopseudomonas sp. OS33-UV13-5 chitinase production displayed a maximum at 9.94±1.08 U/mL on day 9 of cultivation. The accumulation of N-acetyl glucosamine (NAG) was about 1.98±0.32 to 3.38±0.81 mg/L. Future research should emphasize on the fed-batch cultivation to enhance the CD production by maximizing chitin assimilation.
References
Alper, H., Jin, Y.-S., Moxley, J. F., & Stephanopoulos, G. (2005). Identifying gene targets for the metabolic engineering of lycopene biosynthesis in Escherichia coli. Metabolic Engineering, 7(3), 155–164.https://doi.org/10.1016/j.ymben.2004.12.003
Amar, E. C., Kiron, V., Satoh, S., & Watanabe, T. (2003). Enhancement of innate immunity in rainbow trout (Oncorhynchus mykiss Walbaum) associated with dietary intake of carotenoids from natural products. Fish and Shellfish Immunology, 16, 527–537.
Bell, J. G., McEvoy, J., Tocher, D. R., & Sargent, J. R. (2000). Depletion of α-tocopherol and astaxanthin in Atlantic salmon (Salmo salar) affects autoxidative defense and fatty acid metabolism. The Journal of Nutrition, 130, 1800–1808.
Cohen-Bazire, G., Sistrom, W. R., & Stanier, R. Y. (1957). Kinetic studies of pigment synthesis by non-sulfur purple bacteria. Journal of Cellular Physiology, 49, 25–68.
García-Chavarría, M., & Lara-Flores, M. (2013). The use of carotenoid in aquaculture. Research Journal of Fisheries and Hydrobiology, 8(2), 38–49.
Guan, C., Ji, Y., Hu, J., Hu, C., Yang, F., & Yang, G. (2017). Biotransformation of rutin using crude enzyme from Rhodopseudomonas palustris. Current Microbiology, 74, 431–436. https://doi.org/10.1007/s00284-017-1204-3
Krairak, S. (2023). Production of coenzyme Q10 and carotenoid by cultivation of Rhodopseudomonas sp. OS33-UV13-5 using raw starch as carbon source. Journal of Science Ladkrabang, 32(2), 136–151.
Kuo, F.-S., Chien, Y.-H., & Chen, C.-J. (2012). Effects of light sources on growth and carotenoid content of photosynthetic bacteria Rhodopseudomonas palustris. Bioresource Technology, 113, 315-318.https://doi.org/10.1016/j.biortech.2012.01.087
Larimer, F. W., Chain, P., Hauser, L., Lamerdin, J., Malfatti, S., Do, L., Land, M. L., Pelletier, D. A., Beatty, J. T., Lang, A. S., Tabita, F. R., Gibson, J. L., Hanson, T. E., Bobst, C., Torres, J. L. T., Peres, C., Harrison, F. H., Gibson, J., & Harwood, C. S. (2003). Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nature Biotechnology, 22, 55–61.
Lavall, R. L., Assis, O. B., & Campana-Filho, S. P. (2007). -Chitin from the pens of Loligo sp.: Extraction and characterization. Bioresource Technology, 98(13), 2465–2472.
Li, M., Hou, F., Wu, T., Jiang, X., Li, F., Liu, H., Xian, M., & Zhang, H. (2019). Recent advances of metabolic engineering strategies in natural isoprenoid production using cell factories. Natural Product Reports, 37(1), 80–99.
Li, X. P., Bjorkman, O., Shih, C., Grossman, A. R., Rosenquist, M., Jansson, S., & Niyogi, K. K. (2000). A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature, 403, 391–395.
Lim, K. C., Yusoff, F. M., Shariff, M., & Kamarudin, M. S. (2018). Astaxanthin as feed supplement in aquatic animals. Reviews in Aquaculture, 10(3), 738–773.
Liu, Y. S., & Wu, J. Y. (2007). Optimization of cell growth and carotenoid production of Xanthophyllomyces dendrorhous through statistical experiment design. Biochemical Engineering Journal, 36(2), 182–189.https://doi.org/10.1016/j.bej.2007.02.014
Lopez-Romero, J., Salgado-Manjarrez, E., Torres, L., & Garcia-Pena, E. I. (2020). Enhanced carotenoid production by Rhodopseudomonas palustris ATCC 17001 under low light conditions. Journal of Biotechnology, 323, 159–165. https://doi.org/10.1016/j.jbiotec.2020.08.007
Marđetko, N., Kolakušić, A., Trontel, A., Novak, M., Pavlečić, M., Dobrinčić, A., Tominac, V. P., & Šantek, B. (2025). Usage of the fungus Mucor indicus and the bacterium Rhodovulum adriaticum in a biorefinery system for biochemical production on grass hydrolysates. Polymers, 17(3), 369.https://doi.org/10.3390/polym17030369
Mehrabi, S., Ekanemesang, U. M., Aikhionbare, F. O., Kimbro, K. S., & Bender, J. (2001). Identification and characterization of Rhodopseudomonas spp., a purple, non-sulfur bacterium from microbial mats. Biomolecular Engineering, 18, 49–56.
Minke, R., & Blackwell, J. (1978). The structure of α-chitin. Journal of Molecular Biology, 120(2), 167–181.
Nakano, T. (2003). Microorganisms. In H. Nakagawa & M. Sato (Eds.), Micronutrients and health of cultured fish (pp. 95-106). Koseisha Koseikaku.
Niu, J., Wen, H., Li, C.-H., Liu, Y.-J., Tian, L.-X., Chen, X., Huang, Z., & Lin, H.-Z. (2014). Comparison effect of dietary astaxanthin and β-carotene in the presence and absence of cholesterol supplementation on growth performance, antioxidant capacity and gene expression of Penaeus monodon under normoxia and hypoxia condition. Aquaculture, 422-423, 8–17. https://doi.org/10.1016/j.aquaculture.2013.11.013
Page, G. I., Russell, P. M., & Davies, S. J. (2005). Dietary carotenoid pigment supplementation influences hepatic lipid and mucopolysaccharide levels in rainbow trout (Oncorhynchus mykiss). Comparative Biochemistry and Physiology Part B, 142, 398–402.
Pfennig, N. (1969). Rhodopseudomonas acidophila, sp. n., a new species of the budding purple nonsulfur bacteria. Journal of Bacteriology, 99(2), 597–602.
Ramírez, M. Á., Rodriguez, A. T., Alfonso, L., & Peniche, C. (2010). Chitin and its derivatives as biopolymers with potential agricultural applications. Biotecnología Aplicada, 27, 270–276.
Raposo, M. F. J., De Morais, A. M. M. B., & De Morais, R. M. S. C. (2015). Carotenoids from marine microalgae: A valuable natural source for the prevention of chronic diseases. Marine Drugs, 13(8), 5128–5155.https://doi.org/10.3390/md13085128
Schagerl, M., & Müller, B. (2006). Acclimation of chlorophyll a and carotenoid levels to different irradiances in four freshwater cyanobacteria. Journal of Plant Physiology, 163(7), 709–716.https://doi.org/10.1016/j.jplph.2005.09.015
Schiedt, K., Leuenberger, F. J., Vecchi, M., & Glinz, E. (1985). Absorption, retention and metabolic transformation of carotenoids in rainbow trout, salmon and chicken. Pure and Applied Chemistry, 57(5), 685–692.
Truper, H. G., & Pfennig, N. (1981). Characterization and identification of the anoxygenic phototrophic bacteria. In M. P. Starr, H. Stolp, H. G. Trüper, A. Balows, & H. G. Schlegel (Eds.), The prokaryotes: A handbook on habitats, isolation, and identification of bacteria (pp. 299-312). Springer-Verlag Berlin Heidelberg.
Vassallo-Agius, R., Imaizumi, H., Watanabe, T., Yamazaki, T., Satoh, S., & Kiron, V. (2001). The influences of astaxanthin supplemented dry pellets on spawning of striped jack. Fisheries Sciences, 67, 260–270.
Wang, S.-L., Yang, C.-W., Liang, T.-W., Peng, J.-H., & Wang, C.-L. (2009). Degradation of chitin and production of bioactive materials by bioconversion of squid pens. Carbohydrate Polymer, 78, 205–212.https://doi.org/10.1016/j.carbpol.2009.03.020
Zhou, X., Tian, Z., Wang, Y., & Li, W. (2010). Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune response. Fish Physiology and Biochemistry, 36(3), 501–509. https://doi.org/10.1007/s10695-009-9320-z
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Suan Sunandha Rajabhat University

This work is licensed under a Creative Commons Attribution 4.0 International License.











