Study on Intracellular Extracts of Chlorella sp. KLSc61 to Promote Growth of Lactobacillus plantarum JCM 1149
DOI:
https://doi.org/10.53848/ssstj.v12i1.1022Keywords:
Chlorella sp. KLSc61, Ethanol, Intracellular extract, Lactobacillus plantarumAbstract
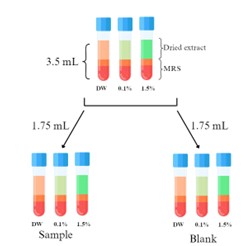
Chlorella is one of the famous microalga genera used to study in various fields, including functional food. Several studies revealed that its dried biomass could be used as prebiotics to promote the growth of probiotics such as Lactobacillus sp. Using Chlorella dried cell mass might be ineffective while producing on an industrial scale. To find an alternative strategy, we tested if Chlorella crude extract comprised some bioactive compound that could promote the growth of Lactobacillus or not, this led to reduce the amount of Chlorella use. This study aimed to compare fresh and dried Chlorella crude extract to find out which one could be a better use, which was still unclear. We prepared fresh and dried intracellular ethanolic extracts of Chlorella sp. KLSC61, added them into the Lactobacillus plantarum JCM 1149 growth medium (MRS), and measured the growth of L. plantarum JCM 1149. The results showed that all dried crude extracts significantly showed better L. plantarum JCM 1149 growth promotion than fresh ones. The 0.1% (w/v) of dried-ethanolic crude extract could promote growth up to 1.1-1.2-fold compared to controls (MRS and glucose) at 14 and 24 h during the stationary phase. These preliminary results demonstrated that Chlorella sp. KLSC61 crude extracts contained some bioactive compounds that could enhance the growth of L. plantarum JCM 1149, and that compounds might be heat resistant. We propose analyzing the crude extract and finding out a specific compound that could benefit the growth of L. plantarum JCM 1149 for future study.
References
Abdel-Raouf, N., Al-Homaidan, A. A., & Ibraheem, I. B. M. (2012). Microalgae and wastewater treatment.Saudi Journal of Biological Sciences, 19(3), 257–275. https://doi.org/10.1016/j.sjbs.2012.04.005
Abreu, A. P., Martins, R., & Nunes, J. (2023). Emerging applications of Chlorella sp. and Spirulina (Arthrospira) sp. Bioengineering, 10(8), 955. https://doi.org/10.3390/bioengineering10080955
Andrade, L. M., Andrade C. J., Dias, M., Nascimento, C. A., & Mendes, M. A. (2018). Chlorella and Spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements; An overview. MOJ Food Processing & Technology, 6(1),45-58. https://doi.org/10.15406/mojfpt.2018.06.00144
Babich, O., Sukhikh, S., Larina, V., Kalashnikova, O., Kashirskikh, E., Prosekov, A., Noskova, S., Ivanova, S., Fendri, I., Smaoui, S., Abdelkafi, S., Michaud, P., & Dolganyuk, V. (2022). Algae: Study of edible and biologically active fractions, their properties and applications. Plants, 11(6),780. https://doi.org/10.3390/plants11060780
Bernaerts, T. M. M., Gheysen, L., Kyomugasho, C., Kermani, Z. J., Vandionant, S., Foubert, I., Hendrickx, M. E., & Van Loey, A. M. (2018). Comparison of microalgal biomasses as functional food ingredients: Focus on the composition of cell wall related polysaccharides. Algal Research, 32, 150–161. https://doi.org/10.1016/j.algal.2018.03.017
Bito, T., Okumura, E., Fujishima, M., & Watanabe, F. (2020). Potential of Chlorella as a dietary supplement to promote human health. Nutrients, 12(9), 2524. https://doi.org/10.3390/nu12092524
Gao, L., Qin, Y., Zhou, X., Jin, W., He, Z., Li, X., & Wang, Q. (2024). Microalgae as future food: Rich nutrients, safety, production costs and environmental effects. Science of the Total Environment, 927, 172167.https://doi.org/10.1016/j.scitotenv.2024.172167
Georgiopoulou, I., Tzima, S., Pappa, G. D., Louli, V., Voutsas, E., & Magoulas, K. (2021). Experimental design and optimization of recovering bioactive compounds from Chlorella vulgaris through conventional extraction. Molecules, 27(1), 29. https://doi.org/10.3390/molecules27010029
Gerde, J. A., Montalbo-Lomboy, M., Yao, L., Grewell, D., & Wang, T. (2012). Evaluation of microalgae cell disruption by ultrasonic treatment. Bioresource Technology, 125, 175–181. https://doi.org/10.1016/j.biortech.2012.08.110
Goiris, K., Muylaert, K., Fraeye, I., Foubert, I., De Brabanter, J., & De Cooman, L. (2012). Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. Journal of Applied Phycology, 24(6), 1477–1486. https://doi.org/10.1007/s10811-012-9804-6
Gorman, D. S., & Levine, R. P. (1965). Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proceedings of the National Academy of Sciences of the United States of America, 54(6), 1665–1669. https://doi.org/10.1073/pnas.54.6.1665
Hadiatullah, H., Wang, H., Liu, Y.-X., & Fan, Z.-C. (2020). Chlamydomonas reinhardtii-derived multimer Mytichitin-CB possesses potent antibacterial properties. Process Biochemistry, 96, 21–29. https://doi.org/10.1016/j.procbio.2020.05.010
Ilieva, Y., Zaharieva, M. M., Kroumov, A. D., & Najdenski, H. (2024). Antimicrobial and ecological potential of Chlorellaceae and Scenedesmaceae with a focus on wastewater treatment and industry. Fermentation, 10(7), 341. https://doi.org/10.3390/fermentation10070341
Laokua, N., Rittiyan, N., Kornrawudaphikasama, Y., Klinsalee, R., Tonawut, Y., Preechaphonkul, N., Raksajit, W., Khetkorn, W., Dejtisakdi, W., & Maneeruttanarungroj, C. (2022). Optimal conditions for maximized H2 yield from a new green algal strain Chlorella sp. KLSc61. Journal of Applied Phycology, 34(4), 1909–1919. https://doi.org/10.1007/s10811-022-02779-y
Liu, Y.-X., Li, Z.-F., Lv, Y.-J., Dong, B., & Fan, Z.-C. (2020). Chlamydomonas reinhardtii-expressed multimer of Bacteriocin LS2 potently inhibits the growth of bacteria. Process Biochemistry, 95, 139–147. https://doi.org/10.1016/j.procbio.2020.05.024
Lv, K., Yuan, Q., Li, H., Li, T., Ma, H., Gao, C., Zhang, S., Liu, Y., & Zhao, L. (2022). Chlorella pyrenoidosa polysaccharides as a prebiotic to modulate gut microbiota: Physicochemical properties and fermentation characteristics in vitro. Foods, 11(5), 725. https://doi.org/10.3390/foods11050725
Rizwan, M., Mujtaba, G., Memon, S. A., Lee, K., & Rashid, N. (2018). Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renewable and Sustainable Energy Reviews, 92, 394–404. https://doi.org/10.1016/j.rser.2018.04.034
Safafar, H., van Wagenen, J., Møller, P., & Jacobsen, C. (2015). Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Marine Drugs, 13(12), 7339–7356. https://doi.org/10.3390/md13127069
Safi, C., Zebib, B., Merah, O., Pontalier, P.-Y., & Vaca-Garcia, C. (2014). Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renewable and Sustainable Energy Reviews, 35, 265–278. https://doi.org/10.1016/j.rser.2014.04.007
Ścieszka, S., & Klewicka, E. (2020). Influence of the microalga Chlorella vulgaris on the growth and metabolic activity of Lactobacillus spp. bacteria. Foods, 9(7), 959. https://doi.org/10.3390/foods9070959
Smith, A. M., & Zeeman, S. C. (2020). Starch: A flexible, adaptable carbon store coupled to plant growth. Annual Review of Plant Biology, 71, 217–245. https://doi.org/10.1146/annurev-arplant-050718-100241
Sylwia, Ś., & Elżbieta, K. (2020). Algae Chlorella vulgaris as a factor conditioning the survival of Lactobacillus spp. in adverse environmental conditions. LWT - Food Science and Technology, 133, 109936. https://doi.org/10.1016/j.lwt.2020.109936
Tiwari, S., & Dhakal, N. (2023). Analysis of variations in biomolecules during various growth phases of freshwater microalgae Chlorella sp. Applied Food Biotechnology, 10(1), 73–84. https://doi.org/10.22037/afb.v10i1.39796
Wan, P., Liu, H., Ding, M., Zhang, K., Shang, Z., Wang, Y., & Ma, Y. (2023). Physicochemical characterization, digestion profile and gut microbiota regulation activity of intracellular polysaccharides from Chlorella zofingiensis. International Journal of Biological Macromolecules, 253(Pt. 3), 126881. https://doi.org/10.1016/j.ijbiomac.2023.126881
Xue, B., Dong, C.-M., Hu, H.-H., Dong, B., & Fan, Z.-C. (2020). Chlamydomonas reinhardtii-expressed multimer of ToAMP4 inhibits the growth of bacteria of both Gram-positive and Gram-negative. Process Biochemistry, 91, 311–318. https://doi.org/10.1016/j.procbio.2020.01.001
Yu, M., Chen, M., Gui, J., Huang, S., Liu, Y., Shentu, H., He, J., Fang, Z., Wang, W., & Zhang, Y. (2019). Preparation of Chlorella vulgaris polysaccharides and their antioxidant activity in vitro and in vivo. International Journal of Biological Macromolecules, 137, 139 –150. https://doi.org/10.1016/j.ijbiomac.2019.06.222
Zavizion, B., Zhao, Z., Nittayajarn, A., & Rieder, R. J. (2010). Rapid microbiological testing: Monitoring the development of bacterial stress. PLoS One, 5(10), e13374. https://doi.org/10.1371/journal.pone.0013374
Zheng, L., Oh, S. T., Jeon, J. Y., Moon, B. H., Kwon, H. S., Lim, S. U., An, B. K., & Kang, C. W. (2012). The dietary effects of fermented Chlorella vulgaris (CBT®) on production performance, liver lipids and intestinal microflora in laying hens. Asian-Australasian Journal of Animal Sciences, 25(2), 261–266.https://doi.org/10.5713/ajas.2011.11273
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Suan Sunandha Rajabhat University

This work is licensed under a Creative Commons Attribution 4.0 International License.











