Bio-based Polyurethane Derived from Carbon Dioxide and Epoxidized Soybean Oil
DOI:
https://doi.org/10.53848/ssstj.v9i2.229Keywords:
Non-isocyanate polyurethanes, Bio-based polyurethane, Epoxidized soybean oil, BioadhesivesAbstract
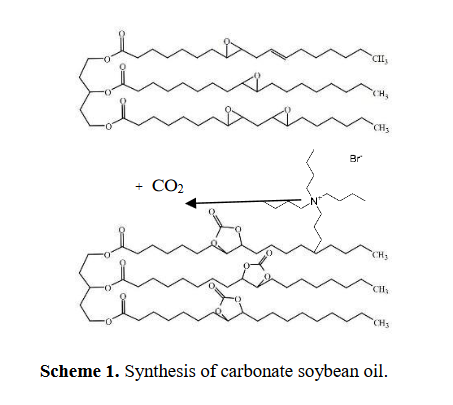
The synthesis of polyurethane relies on a toxic and petroleum-based isocyanate reactant. The aim of this research was to synthesize Polyurethane using environment-benign and renewable starting materials such as carbon dioxide and soybean oil. The carbonated soybean oil was first prepared from carbon dioxide (CO2) and epoxidized soybean oil (ESBO) using zinc glutarate (ZnGA) as a catalyst but the result of FTIR indicated the absence of the peak of cyclic carbonate around 1800 cm-1. Therefore, in this work, the synthesis of Polyurethane was modified from A. Lee (Lee & Deng, 2015) using tetramethylammonium bromide (TBAB) as a catalyst. The as-synthesized carbonated soybean oil (CSBO) was allowed to react with two types of substances, 3-aminopropyltriethoxysilane or diethylenetriamine with the molar ratios of cyclic carbonate:NH2 of 1:1 with THF or DMF as solvents to obtain Polyurethanes (U1THF, U1DMF, U2THF, U2DMF). After 3 hours lignin solution was added to form a film. Raman spectra confirmed the catalyst removal from CSBO. FTIR spectra showed the peak around 1800 cm-1 assigned to cyclic carbonate of CSBO, and a new peak of urethane linkage around 1700 cm-1 (C=O stretching) of Polyurethanes. The conversion of epoxide to cyclic carbonate was also confirmed by 1H-NMR. Upon adding lignin into the Polyurethanes, the lignin-urethane U1THF, and U1DMF formed films whereas U2THF, and U2DMF formed viscous liquids. In terms of applications, all four formulations can be potentially applied as bioadhesives.
References
Caraculacu, A. A., & Coseri, S. (2001). Isocyanates in polyaddition processes. Structure and reaction mechanisms. Progress in Polymer Science, 26, 799-851.
Chunsakul, T., Seadan, M., & Suttiruengwong, S. (2019). Copolycarbonate derived from
carbon dioxide as toughness improvement for Poly (lactic acid). Materials Today: Proceedings, 17, 1949-1955. doi:10.1016/j.matpr.2019.06.239
Cornille, A., Auvergne, R., Figovsky, O., Boutevin, B., & Caillol, S. (2017). A perspective approach to sustainable routes for nonisocyanate polyurethanes. European Polymer Journal, 87, 535-552. doi:10.1016/j.eurpolymj.2016.11.027
Doll, K. M., & Erhan, S. Z. (2005). The improved synthesis of carbonated soybean oil using supercritical carbon dioxide at a reduced reaction time. Green Chemistry, 7(12), 849-854. doi:10.1039/b511014a
González Martínez, D. A., Vigueras Santiago, E., & Hernández López, S. (2021). Yield and selectivity improvement in the synthesis of carbonated linseed oil by catalytic conversion of carbon dioxide. Polymers, 13(6). doi:10.3390/polym13060852
Gupta, S., & Upadhyaya, R. (2014). Cancer and p21 protein expression: In relation with isocyanate toxicity in cultured mammalian cells. The International Journal of Pharmaceutical Research and BioSciences, 3(6), 20-28.
Javni, I., Hong, D. P., & Petrović, Z. S. (2008). Soybased polyurethanes by nonisocyanate route. Journal of Applied Polymer Science, 108(6), 3867-3875. doi:10.1002/app.27995
Kathalewar, M. S., Joshi, P. B., Sabnis, A. S., & Malshe, V. C. (2013). Non-isocyanate polyurethanes: From chemistry to applications. RSC Advances, 3(13), 4110- 4129. doi:10.1039/c2ra21938g
Lee, A., & Deng, Y. (2015). Green polyurethane from lignin and soybean oil through nonisocyanate reactions. European Polymer Journal, 63, 67-73. doi:10.1016/j.eurpolymj.2014.11.023
Maisonneuve, L., Lamarzelle, O., Rix, E., Grau, E., & Cramail, H. (2015). Isocyanate-free routes to polyurethanes and poly(hydroxy urethane)s. Chemical Reviews, 115(22), 12407-12439. doi:10.1021/acs.chemrev.5b00355
Pfister, D. P., Xia, Y., & Larock, R. C. (2011). Recent advances in vegetable oil-based polyurethanes. ChemSusChem, 4(6), 703-717. doi:10.1002/cssc.201000378
Ree, M., Bae, J. Y., Jung, J. H., & Shin, T. J. (1999). A new copolymerizayion processs leading to poly(propylene carbonate) with a highly enhanced yield from carbon dioxide and propylene oxide. Journal of Polymer Science Part A: Polymer Chemistry, 37(12), 1863-1876.
Robles, E., Csóka, L., & Labidi, J. (2018). Effect of reaction conditions on the surface modification of cellulose nanofibrils with aminopropyl triethoxysilane. Coatings, 8(4). doi:10.3390/coatings8040139
Rokicki, G., Parzuchowski, P. G., & Mazurek, M. (2015). Non-isocyanate polyurethanes: Synthesis, properties, and applications. Polymers for Advanced Technologies, 26(7), 707-761. doi:10.1002/pat.3522
Rokicki, G., & Piotrowska, A. (2002). A new route to polyurethanes from ethylene carbonate, diamines and diols. Polymer, 43, 2927-2935.
Taherimehr, M., & Pescarmona, P. P. (2014). Green polycarbonates prepared by the copolymerization of CO2 with epoxides. Journal of Applied Polymer Science, 131(21). doi:10.1002/app.41141