The Effect of Extraction Methods on Phenolic, Anthocyanin, and Antioxidant Activities of Riceberry Bran
Keywords:
Accelerated solvent extraction, Maceration, Riceberry bran, Soxhlet, Ultrasonic-assisted extractionAbstract
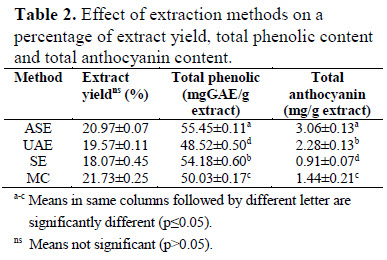
Riceberry bran, byproduct from the rice milling process, is one of bioactive compounds sources. In order to increase Riceberry bran value, the purpose of this study was to compare the effect of different Riceberry bran extraction methods, including accelerated solvent extraction (ASE), ultrasonic-assisted extraction (UAE), soxhlet extraction (SE), and maceration method (MC) on bioactive compounds and antioxidant activities. It was found that the Riceberry bran extract from ASE presented the highest total phenolic, total anthocyanin content (55.45 mg GAE/g and 3.06 mg/g) and antioxidant properties including IC50 of DPPH assay (0.109 mg/mL),
FRAP value (688.04 mmole Fe(II)/kg) and IC50 of ABTS assay (3.42 mg/mL) among the four methods. These results imply the potential to use the ASE method for extraction of phenolic and anthocyanin compounds from Riceberry bran.
References
Abdel-Aal, E., Akhtar, H., Rabalski, I., & Bryan, M. (2014). Accelerated, microwave-assisted, and conventional solvent extraction methods
affect anthocyanin composition from colored grains. Journal of Food Science, 79(2), C138-C146.
Association of Official Analytical Chemists. (2019). Official methods of analysis (21st ed.). Washington DC: Author.
Barros, F., Dykes, L., Awika, J. M., & Rooney, L. W. (2013). Accelerated solvent extraction of phenolic compounds from sorghum brans.
Journal of Cereal Science, 58(2), 305-312.
Benzie, I. F. F., & Strain, J. J. (1999). Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods in Enzymology, 299,
-27.
Cacace, J. E., & Mazza, G. (2003). Optimization of extraction of anthocyanins from black currants with aqueous ethanol. Journal of Food
Science, 68(1), 240-248.
Chen, X. Q., Nagao, N., Itani, T., & Irifune, K. (2012). Anti-oxidative analysis, and identification and quantification of anthocyanin pigments in different coloured rice. Food Chemistry, 135(4), 2783-2788.
Das, A. B., Goud, V. V., & Das, C. (2017). Extraction of phenolic compounds and anthocyanin from black and purple rice bran (Oryza sativa L.) using ultrasound: A comparative analysis and phytochemical profiling. Industrial Crops and Products, 95, 332-341.
Dudonne, S., Vitrac, X., Coutiere, P., Woillez, M., & Merillon, J. (2009). Comparative study of antioxidant properties and total phenolic content
of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. Journal of Agricultural and Food Chemistry,57(5),
-1774
Giusti, M. M., & Wrolstad, R. E. (2005). Characterization and measurement of anthocyanins by UV-visible spectroscopy. Current Protocols in Food Analytical Chemistry, 1, F1.2.1-F1.2.13.
Ju, Z. Y., & Howard, L. R. (2003). Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics
from dried red grape skin. Journal of Agricultural and Food Chemistry, 51, 5207-5213.
Leardkamolkarn, V., Thongthep, W., Suttiarporn, P., Kongkachuichai, R., Wongpornchai, S., & Wanavijitr, A. (2011). Chemopreventive properties of the bran extracted from a newly developed Thai rice: The Riceberry. Food Chemistry, 125(3), 978-985. doi:10.1016/j.foodchem.2010.09.093
Peanparkdee, M., Yamauchi, R., & Iwamoto, S. (2018). Characterization of antioxidants extracted from Thai Riceberry bran using ultrasonic-assisted and conventional solvent extraction methods. Food and Bioprocess Technology, 11, 713-722.
Posuwan, J., Prangthip, P., Leardkamolkarn, V., Yamborisut, U., Surasiang, R., Charoensiri, R., & Kongkachuichai, R. (2013). Long-term supplementation of high pigmented rice bran oil (Oryza sativa L.) on amelioration of oxidative stress and histological changes in streptozotocin-induced diabetic rats fed a high fat diet; Riceberry bran oil. Food Chemistry, 138(1), 501-508.
Prangthip, P., Surasiang, R., Charoensiri, R., Leardkamolkarn, V., Komindr, S., Yamborisut, U. … Kongkachuichai, R. (2013). Amelioration of hyperglycemia, hyperlipidemia, oxidative stress and inflammation in steptozotocininduced diabetic rats fed a high fat diet by Riceberry supplement. Journal of Functional Foods, 5, 195-203.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26 (9-10), 1231-1237.
Soradech, S., Reungpatthanaphong, P., Tangsatirapakdee, S., Panaphong, K., Thammachat, T., Manchun, S., & Thubthimthed, S. (2016). Radical scavenging, antioxidant and melanogenesis stimulating activities of different species of rice (Oryza sativa L.) extracts for hair treatment
formulation. Thai Journal of Pharmaceutical Science, 40, 92-95.
Sripum, C., Kukreja, R. K., Charoenkiatkul, S., Kriengsinyos, W., & Suttisansanee, U. (2017). The effect of extraction conditions on antioxidant activities and total phenolic contents of different processed Thai Jasmine rice. International Food Research Journal,
(4), 1644-1650.
Suttiarporn, P., Sookwong, P., & Mahatheeranont, S. (2016). Fractionation and identification of antioxidant compounds from bran of Thai
Black Rice cv. Riceberry. International Journal of Chemical Engineering and Applications, 7(2), 109-114.
Tabaraki, R., & Nateghi, A. (2011). Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrasonics Sonochemistry, 18(6), 1279-1286.
Tao, Y., Wu, D., Zhang, Q., & Sun, D. (2014). Ultrasound-assisted extraction of phenolics from wine lees: Modeling, optimization and stability of extracts during storage. Ultrasonics Sonochemistry, 21(2), 706-715.
Wolfe, K., Wu, X., & Liu, R. H. (2003). Antioxidant activity of apple peels. Journal of Agricultural and Food Chemistry, 51, 609-614.