Electrospun Cellulose Acetate/Maleic Acid Functionalized Gold Nanoparticles as Pb(II) Ions Colorimeter Senser Strip
DOI:
https://doi.org/10.53848/ssstj.v11i2.764Keywords:
Gold nanoparticle, Lead detection, Electrospinning process, Colorimetric sensor, Sensor stripAbstract
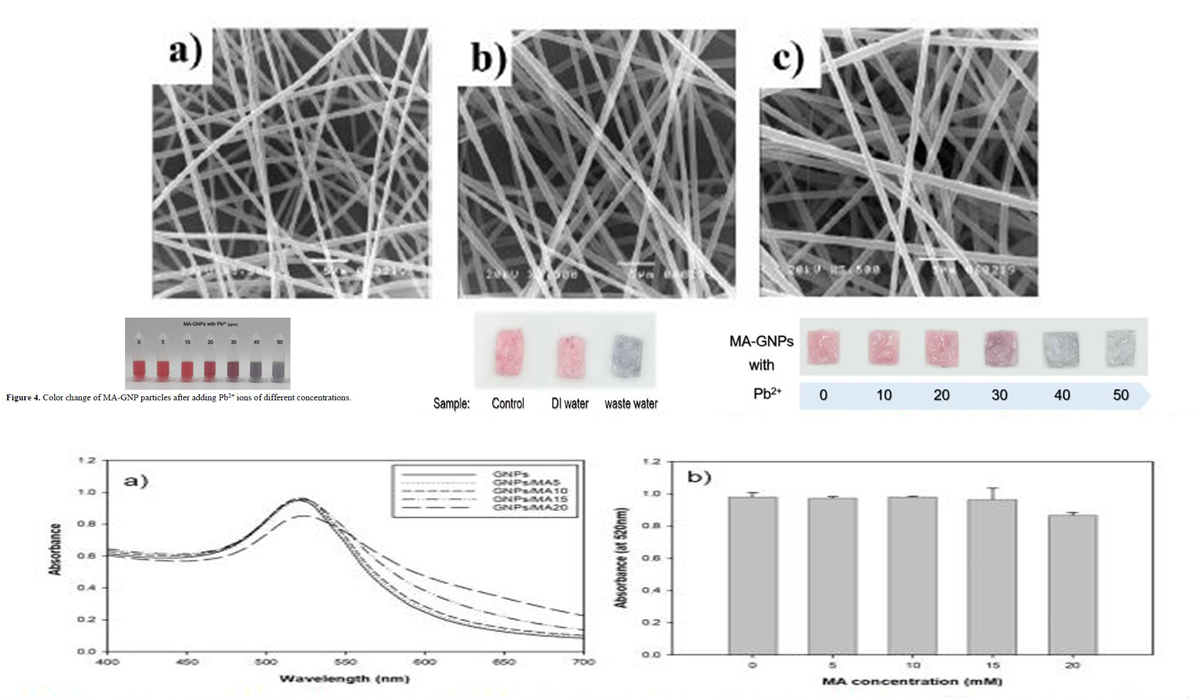
This study investigates the synthesis of gold nanoparticles and surface modifications to enable their utilization as a colorimetric sensor strip on electrospun nanofibrous. Additionally, it utilizes cellulose acetate electrospun fiber preparations as substrates for loading the gold nanoparticles. In order to produce gold nanoparticles, citrate reduction was employed, followed by modification with maleic acid (MA-GNPs). MA-GNPs were significantly more selective for Pb2+ than for other ions (Co2+, Cu2+, Hg2+, Ni+), according to the results. Following the introduction of Pb2+, the solutions exhibited a color change from red to blue or purple, which was attributed to the aggregation of nanoparticles, as determined by UV-Vis spectrometry. An optical band indicative of GNPs and MA-GNPs was detected at an estimated wavelength of 520 nm. Approximately 600 nanometers after the addition of Pb2+, the intensity of the solution progressively decreased to 520 nanometers, and a new band emerged. To fabricate cellulose acetate nanofibrous via electrospinning, the impact of solvent ratios, polymer concentrations, and process conditions were investigated. The ideal parameters for the fabrication of the substrate were as follows: 15%w/w polymer solution, 15 kV electrospinning voltage, 15 cm needle-to-collector distance, and 48 hours of collecting time. In order to examine the color change of the prepared strip, it is observed that the strip transforms from red to blue upon exposure to Pb2+ ions of varying concentrations, just as it does in solution form. An examination of water samples indicated that the strip remained colorless when tested with DI water. However, testing with wastewater sourced from a battery facility identified a transformation of the strip from red to purplish blue. This is equivalent to an estimated lead concentration of 40 parts per million. The outcomes showcased the capacity of the discolored strips to identify the preliminary concentration of lead ions, suggesting potential for future advancements in this area.
References
References
Abdullah, N., Yusof, N., Lau, W. J., Jaafar, J., & Ismail, A. F. (2019). Recent trends of heavy metal removal from water/wastewater by membrane technologies. Journal of Industrial and Engineering Chemistry, 76, 17-38. doi:10.1016/j.jiec.2019.03.029
Chansuwan, W. (2022). The design of chemosensors for naked-eye detection of mercury (II) ion. KKU Science Journal, 42(4), 748-760. Retrieved from https://ph01.tci-thaijo.org/index.php/KKUSciJ/article/view/249317
Chen, J. P., & Wang, L. (2004) Characterization of metal adsorption kinetic properties in batch and fixed-bed reactors. Chemosphere, 54, 397-404. doi:10.1016/S0045-6535(03)00714-8
Ding, N., Cao, Q., Zhao, H., Yang, Y., Zeng, L., He, Y., … Wang, G. (2010). Colorimetric assay for determination of lead (II) based on its incorporation into gold nanoparticles during their synthesis. Sensors, 10, 11144-11155. doi:10.3390/s101211144
Feng, X., Long, R., Wang, L., Liu, C., Bai, Z., & Liu, X. (2022). A review on heavy metal ions adsorption from water by layered double hydroxide and its composites. Separation and Purification Technology, 284, 120099. doi:10.1016/j.seppur.2021.120099
Fewtrell, L., Kaufmann, R., & Prüss-Üstün, A. (2003). Lead: Assessing the environmental burden of disease at national and local levels (pp. 5-21). Geneva: World Health Organization.
Ghaedi, M., Ahmadi, F., & Shokrollahi, A. (2007). Simultaneous preconcentration and determination of copper, nickel, cobalt and lead ions content by flame atomic absorption spectrometry. Journal of Hazardous Materials, 142, 272-278. doi:10.1016/j.jhazmat.2006.08.012
Hammami, I., Alabdallah, N. M., Al jomaa, A., & Kamoun, M. (2021). Gold nanoparticles: Synthesis properties and applications. Journal of King Saud University - Science, 33(7), 101560.
doi:10.1016/j.jksus.2021.101560.
Han, R., Zou, W., Zhang, Z., Shi, J., & Yang, J. (2006). Removal of copper(II) and lead(II) from aqueous solution by manganese oxide coated sand: I. Characterization and kinetic study. Journal of Hazardous Materials, 137, 384-395. doi:10.1016/j.jhazmat.2006.02.021
Huang, Z. M., Zhang, Y. Z., Kotaki, M., & Ramakrishna, S. (2003). A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Science and Technology, 63(15), 2223-2253. doi:10.1016/S0266-3538(03)00178-7
Li, Y., Ding, B., Sun, G., Ke, T., Chen, J., Al-Deyab, S. S., & Yu, J. (2014). Solid-phase pink-to-purple chromatic strips utilizing gold probes and nanofibrous membranes combined system for lead (II) assaying. Sensors and Actuators B: Chemical, 204, 673-681. doi:10.1016/j.snb.2014.08.048
Milne, A., Landing, W., Bizimis, M., & Morton, P. (2010). Determination of Mn, Fe, Co, Ni, Cu, Zn, Cd and Pb in seawater using high resolution magnetic sector inductively coupled mass spectrometry (HR-ICP-MS). Analytica Chimica Acta, 665, 200-207. doi:10.1016/j.aca.2010.03.027
Ratnarathorn, N., Chailapakul, O., & Dungchai, W. (2015). Highly sensitive colorimetric detection of lead using maleic acid functionalized gold nanoparticles. Talanta, 132, 613-618. doi:10.1016/j.talanta.2014.10.024
Sartore, L., Barbaglio, M., Borgese, L., & Bontempi, E. (2011). Polymer-grafted QCM chemical sensor and application to heavy metal ions real time detection. Sensors and Actuators B: Chemical, 155, 538-544.
doi:10.1016/j.snb.2011.01.003
World Health Organization. (2004). Guidelines for drinking – Water quality (3rd ed.). Geneva: WHO.
Zhang, N., Wang, X., Ma, C., Qiao, Y., Zhang, H., Shi, C., … Zhong, J. (2019). Electrospun nanofibrous cellulose acetate/curcumin membranes for fast detection of Pb ions. Journal of Nanoscience and Nanotechnology, 19, 670-674. doi:10.1166/jnn.2019.15893
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Suan Sunandha Rajabhat University

This work is licensed under a Creative Commons Attribution 4.0 International License.











