Preparation, Characterization and Antiradical Activity of Zinc Oxide Nanoparticles
DOI:
https://doi.org/10.53848/ssstj.v9i2.225Abstract
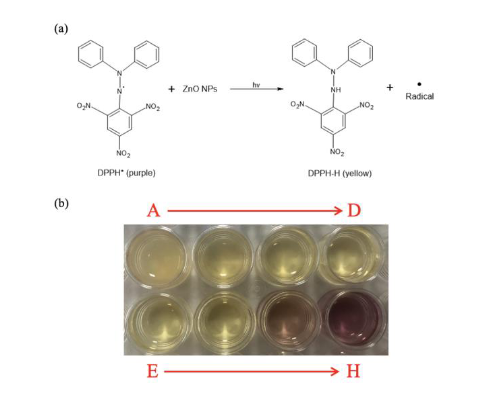
Zinc oxide nanoparticles (ZnO NPs) have recently been studied as a multi-functional and multi-target nanomedicine for cancer treatment. They can be used not only as a nanocarrier for delivery of the chemotherapy drug but also as an antiradical agent due to their photo-catalytic and photo-oxidizing abilities. Our previous work showed a potential use of commercial-available ZnO NPs without and with carboplatin for the treatment of retinoblastoma. The aim of this work was to synthesize ZnO NPs having smaller particle size than the commercial ones, i.e., 100 nm average diameter, in order to improve the reaction time. ZnO NPs were prepared by a sol-gel technique and calcined with different calcination conditions. The structure and particle size of ZnO powders were characterized using an x-ray diffractometer and a particle size analyzer. Average nanoparticle sizes of 16.32 ± 1.64 nm were achieved at a calcination temperature of 300 degree Celsius and 1 hour holding time. The antiradical activity of prepared ZnO NPs in cooperation with ultraviolet irradiation was assessed using a putative model of cancer cells, i.e., 2,2(diphenyl-1-picryhydrazyl) radicals (DPPH*). An optical spectroscopy was used to detect the decrease in peak absorbance of the antiradical solution at a wavelength of 515 nm, which in turn can be used to calculate the percent remaining of DPPH*. The disappearance of DPPH* with respect to the reaction time revealed that prepared ZnO NPs (16.32 ± 1.64 nm) improved response time as compared with ZnO NPs (100 nm). Moreover, the effective ZnO concentrations to reduce the initial DPPH* concentration by 50%, also known as the EC50 value in the present study, is lower indicating the improvement of anti-proliferative activity when compared to the commercial ZnO NPs.
References
Alias, S. S., Ismail, A. B., & Mohamad, A. A. (2010). Effect of pH on ZnO nanoparticle properties synthesized by sol–gel centrifugation. Journal of Alloys and Compounds, 499(2), 231-237. doi:10.1016/j.jallcom.2010.03.174
Aruoma, O. I., & Cuppett, S. L. (Eds.). (1997). Antioxidant methodology: In vivo and in vitro concepts. The American Oil Chemists Society.
Awan, F., Islam, M. S., Ma, Y., Yang, C., Shi, Z., Berry, R. M., & Tam, K. C. (2018). Cellulose nanocrystal–ZnO nanohybrids for controlling photocatalytic activity and UV protection in cosmetic formulation. ACS Omega, 3(10), 12403-12411. doi:10.1021/acsomega.8b01881
Bhati, V. S., Hojamberdiev, M., & Kumar, M. (2020). Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Reports, 6, 46-62. doi:10.1016/j.egyr.2019.08.070
Bisht, G. , & Rayamajhi, S. ( 2016) . ZnO nanoparticles: A promising anticancer agent. Nanobiomedicine, 3. doi:10.5772/63437
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology, 28(1), 25-30. doi:10.1016/S0023-6438(95)80008-5
Chen, P., Wang, H., He, M., Chen, B., Yang, B., & Hu, B. (2019). Size-dependent cytotoxicity study of ZnO nanoparticles in HepG2 cells. Ecotoxicology and Environmental Safety, 171, 337-346. doi:10.1016/j.ecoenv.2018.12.096
da Silva, B. L., Caetano, B. L., Chiari-Andréo, B. G., Pietro, R. C. L. R., & Chiavacci, L. A. (2019). Increased antibacterial activity of ZnO nanoparticles: Influence of size and surface modification. Colloids and Surfaces B: Biointerfaces, 177, 440-447. doi:10.1016/j.colsurfb.2019.02.013
Devi, P. G., & Velu, A. S. (2016). Synthesis, structural and optical properties of pure ZnO and Co doped ZnO nanoparticles prepared by the co-precipitation method. Journal of Theoretical and Applied Physics, 10(3), 233- 240. doi:10.1007/s40094-016-0221-0
Kinnunen, S., Lahtinen, M., Arstila, K., & Sajavaara, T. (2021). Hydrogen and deuterium incorporation in ZnO films grown by atomic layer deposition. Coatings, 11(5), 542. doi:10.3390/coatings11050542
Manikandan, B., Endo, T., Kaneko, S., Murali, K. R., & John, R. (2018). Properties of sol gel synthesized ZnO nanoparticles. Journal of Materials Science: Materials in Electronics, 29(11), 9474-9485. doi:10.1007/s10854-018-8981-8
Marin-Flores, C. A., Rodríguez-Nava, O., GarcíaHernández, M., Ruiz-Guerrero, R., JuárezLópez, F., & Morales-Ramírez, A. D. J. (2021). Free-radical scavenging activity properties of ZnO sub-micron particles: Size effect and kinetics. Journal of Materials Research and Technology, 13, 1665-1675. doi:10.1016/j.jmrt.2021.05.050
Martínez-Carmona, M., Gun’ko, Y., & Vallet-Regí, M. (2018). ZnO nanostructures for drug delivery and theranostic applications. Nanomaterials, 8(4), 268. doi:10.3390/nano8040268
Mishra, P. K., Mishra, H., Ekielski, A., Talegaonkar, S., & Vaidya, B. (2017). Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discovery Today, 22(12), 1825-1834. doi:10.1016/j.drudis.2017.08.006
Narayana, A., Bhat, S. A., Fathima, A., Lokesh, S. V., Surya, S. G., & Yelamaggad, C. V. (2020). Green and low-cost synthesis of zinc oxide nanoparticles and their application in transistor-based carbon monoxide sensing. RSC Advances, 10(23), 13532-13542. doi:10.1039/D0RA00478B
Nguyen, N. T., Nguyen, T. M. N., Le, N. T., & Le, T. K. (2020). Suppressing the photocatalytic activity of ZnO nanoparticles by Al-doping for the application in sunscreen products. Materials Technology, 35(6), 349-355. doi:10.1080/10667857.2019.1684733
Pairoj, S., Damrongsak, P., Damrongsak, B., Jinawath, N., Kaewkhaw, R., Leelawattananon, T., ... & Locharoenrat, K. (2019). Antiradical properties of chemo drug, carboplatin, in cooperation with ZnO nanoparticles under UV irradiation in putative model of cancer cells. Biocybernetics and Biomedical Engineering, 39(3), 893-901. doi:10.1016/j.bbe.2019.08.004
Rahman, F. (2019). Zinc oxide light-emitting diodes: A review. Optical Engineering, 58(1). doi:10.1117/1.OE.58.1.010901
Rani, S., Suri, P., Shishodia, P. K., & Mehra, R. M. (2008). Synthesis of nanocrystalline ZnO powder via sol–gel route for dye-sensitized solar cells. Solar Energy Materials and Solar Cells, 92(12), 1639-1645. doi:10.1016/j.solmat.2008.07.015
Shashanka, R., Esgin, H., Yilmaz, V. M., & Caglar, Y. (2020). Fabrication and characterization of green synthesized ZnO nanoparticle based dyesensitized solar cells. Journal of Science: Advanced Materials and Devices, 5(2), 185-191. doi:10.1016/j.jsamd.2020.04.005
Singh, T. A., Das, J., & Sil, P. C. (2020). Zinc oxide nanoparticles: A comprehensive review on its synthesis, anticancer and drug delivery applications as well as health risks. Advances in Colloid and Interface Science, 286. doi:10.1016/j.cis.2020.102317
Wahab, R., Siddiqui, M. A., Saquib, Q., Dwivedi, S., Ahmad, J., Musarrat, J., ... Shin, H. S. (2014). ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids and Surfaces B: Biointerfaces, 117, 267-276. doi:10.1016/j.colsurfb.2014.02.038
Wallace, R., Brown, A. P., Brydson, R., Wegner, K., & Milne, S. J. (2013). Synthesis of ZnO nanoparticles by flame spray pyrolysis and characterisation protocol. Journal of Materials Science, 48(18), 6393-6403. doi:10.1007/s10853-013-7439-x
Wasly, H. S., Abd El-Sadek, M. S., & Henini, M. (2018). Influence of reaction time and synthesis temperature on the physical properties of ZnO nanoparticles synthesized by the hydrothermal method. Applied Physics A, 124, 1-12. doi:10.1007/s00339-017-1482-4