Crude Extract from Tamarind (Tamarindus indica L.) Husk, Lemongrass (Cymbopogon citratus Stapf.) and Citronella (Cymbopogon nardus Rendle.) Residues Inhibited Seed Germination and Growth of Popping pod (Ruellia tuberosa Linn.)
Keywords:
allelopathy, bioherbicide, methanolic extractAbstract
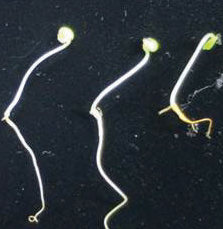
The objective of this research was to investigate the efficacy of extracts from tamarind husk, lemongrass and citronella residues to inhibit seed germination and growth of popping pod. All residues were extracted with water and 80% methanol and subjected to germination and seedling growth bioassay in Petri dish at concentrations of 0, 1.25, 2.5, 5 and 10%. The results showed that aqueous and methanolic extracts from 3 residues could inhibit the germination and growth of popping pod. The crude extract from 80% methanol had higher inhibitory effect on germination and growth than the aqueous extract with more effective inhibition when the concentration had been increased. Methanolic extract from lemongrass and citronella at the concentration of 10% showed the best inhibitory effects. In conclusion, lemongrass and citronella extracts might be a safe alternative for popping pod control to reduce the use of synthetic herbicides.
References
กิติกานต์ ศรีวิชัย. 2554. ปลูกตะไคร้ตัดใบจำหน่ายสร้างรายได้งาม. แหล่งที่มา: http://poonitafarm.blogspot.com/2011/03/blog-post_09.html
อินทิรา ขูดแก้ว, กนกรัตน์ บุญรักษา และปรียานุช สำลี. 2559. ผลของสารสกัดหยาบจากไมยราบและหญ้าขนต่อการงอกและการเติบโตของต้อยติ่ง. แก่นเกษตร 44 ฉ.พิเศษ 1: 777-782.
อมลณัฐ ฉัตรตระกูล. 2555. การพัฒนาผลิตภัณฑ์ปุ๋ยหมักจากวัสดุเหลือใช้ของมะขาม. รายงานวิจัยฉบับสมบูรณ์ ทุนสนับสนุนการวิจัยจากงบประมาณแผ่นดิน มหาวิทยาลัยราชภัฏเพชรบูรณ์ ประจำปี พ.ศ. 2554. แหล่งที่มา: http://research.pcru.ac.th/rdb/pro_data/files/5403002.pdf
Burgos, N.R., R.E. Talbert, K.S. Kim and Y.I. Kuk. 2004. Growth inhibition and root ultrastructure of cucumber seedling exposed to allelochemicals from rye (Secale cereal). J. Chem. Ecol. 30 (3): 671-689.
Dayan, F.E., C.L. Cantrell and S.O. Duke. 2009. Natural products in crop protection. Bioorg. Med. Chem. 17: 4022–4034.
Erez, M.E. and M. Fidan. 2015. Allelopathic effects of sage (Salvia macrochlamys) extract on germination of Portulaca oleracea seeds. Allelopathy J. 35 (2): 285-296.
Farooq, M., K. Jabran, Z.A. Cheema, A. Wahid and K.H.M. Siddique. 2011. The role of allelopathy in agricultural pest management. Pest Manag. Sci. 67: 493-506.
Fujii, Y., T. Shibuya, K. Nakatani, T. Itani, S. Hiradate and M.M. Parvez. 2004. Assessment method for allelopathic effect from leaf litter leachates. Weed Biol. Manag. 4: 19–23.
Fujii, Y. 2009. Overview of research on allelochemicals. W3-01, pp. 1-4. In: Macro Symposium: Challenges for Agro-Environmental Research in Monsoon Asia 5-7 October 2009. National Institute of Agro-Environmental Sciences (NIAES), Ibaraki, Japan.
Kathiresan, R. 2012. Utility tag, farming elements and itk for sustainable management of weeds in changing climate. Pak. J. Weed Sci. Res. 18: 271-282.
Kaur, S., H.P. Singh, D.R. Batish and R.K. Kohli. 2012. Artemisia scoparia essential oil inhibited root growth involves reactive oxygen species (ROS)-mediated disruption of oxidative metabolism: In vivo ROS detection and alterations in antioxidant enzymes. Biochem. Syst. Ecol. 44: 390–399.
Krenchinski, F.H., L.P. Albrecht, A.J.P. Albrecht, P.C. Zonetti, A. Tessele, A.A.M. Barroso and H.F. Placido. 2017. Allelopathic potential of Cymbopogon citratus over beggarticks (Bidens sp.) germination. AJCS 11 (03): 277-283.
Li, Z., Q. Wang, X. Ruan, C. Pan and D. Jiang. 2010. Phenolics and plant allelopathy. Molecules 15: 8933-8952.
Lin, W.X., K.U. Kim and D.H. Shin. 2000. Rice allelopathic potential and its modes of action on Barnyardgrass (Echinochloa crus-galli). Allelopathy J. 7 (2): 215-224.
Putnam, A.R. 1988. Allelochemicals from plants as herbicides. Weed Technol. 2: 510-518.
Soltys, D., A. Rudzin´ska-Langwald, W. Kurek, A. Gniazdowska, E. Sliwinska and R. Bogatek. 2011. Cyanamide mode of action during inhibition of onion (Allium cepa L.) root growth involves disturbances in cell division and cytoskeleton formation. Planta 234: 609–621.
Suwitchayanon, P. and H. Kato-Noguchi. 2014. Allelopathic activity of leaves, stalks and roots of Cymbopogon nardus. Emir. J. Food Agric. 26 (5): 436-443.
Tiwari, P., B. Kumar, M. Kaur, G. Kaur, and H. Kaur. 2011. Phytochemicals screening and extraction: a review. Int. Pharm. Sci. 1 (1): 98-106.
Waris, A., L. Waris, M. A. Khan and A.A. Shad. 2016. Allelopathic effect of methanol and water extracts of Camellia sinensis L. on seed germination and growth of Triticum aestivum L. and Zea mays L. J. Biores. Manag. 3 (1): 1-11.